


Bonding and structure
What you need to know
Reflections and Exam tips
Bonding and structure
Atomic structure and arrangement of electrons
Quick facts:
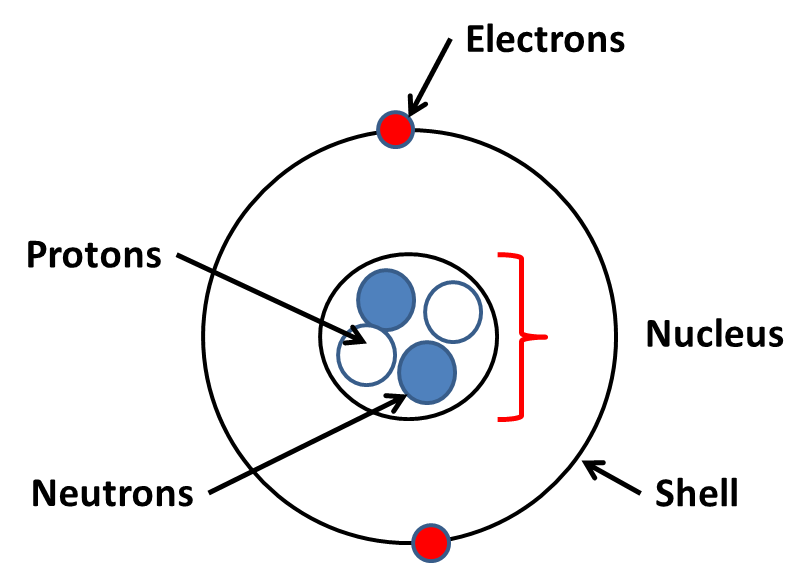
An atoms nucleus contains protons and neutrons. Electrons orbit the nucleus in shells.
The charge of an atom is 0 since it has an equal number of protons and electrons.
| Relative Charge | Relative Mass | Location | |
|---|---|---|---|
| Proton | + | 1 | Nucleus |
| Electron | _ | 1/1840 | Orbit nucleus |
| Neutron | 0 | 1 | Nucleus |
The number of protons in the nucleus is the atomic number or proton number (the bottom number on Periodic Table). The atomic number defines what element it is as its unique for an element.
The atomic model used has electrons arranged round the nucleus in shells, each shell representing a different energy level. The further away the electron, the more energy they have. The electrons are attarcted towards the nucleus by the positively charged protons.
Different energy levels can only hold a certain number of electrons:
First shell = 2 electrons
Second shell = 8 electrons
Third shell = 8 electrons.
Elements with a full outer shell are called noble gases – they are very stable, unreactive and only exist by themselves. For example, argon, neon and helium are noble gases.
In the periodic table:
Rows = Periods
Columns = Groups
Moving along the periods in a Periodic Table, each element has one more electron in their highest energy shell. In each group, all the atoms have the same number of electrons in their highest energy level giving them similar chemical properties.
Why do elements bond?
When atoms react they do so to get a full outer shell (stable octet configuration). This can be done through either:
- Covalent bonding – sharing of electrons
- Ionic bonding – transferring electrons (lose or gain)
Ionic bonding
In ionic bonding, the atoms become charged particles called ions. For example:
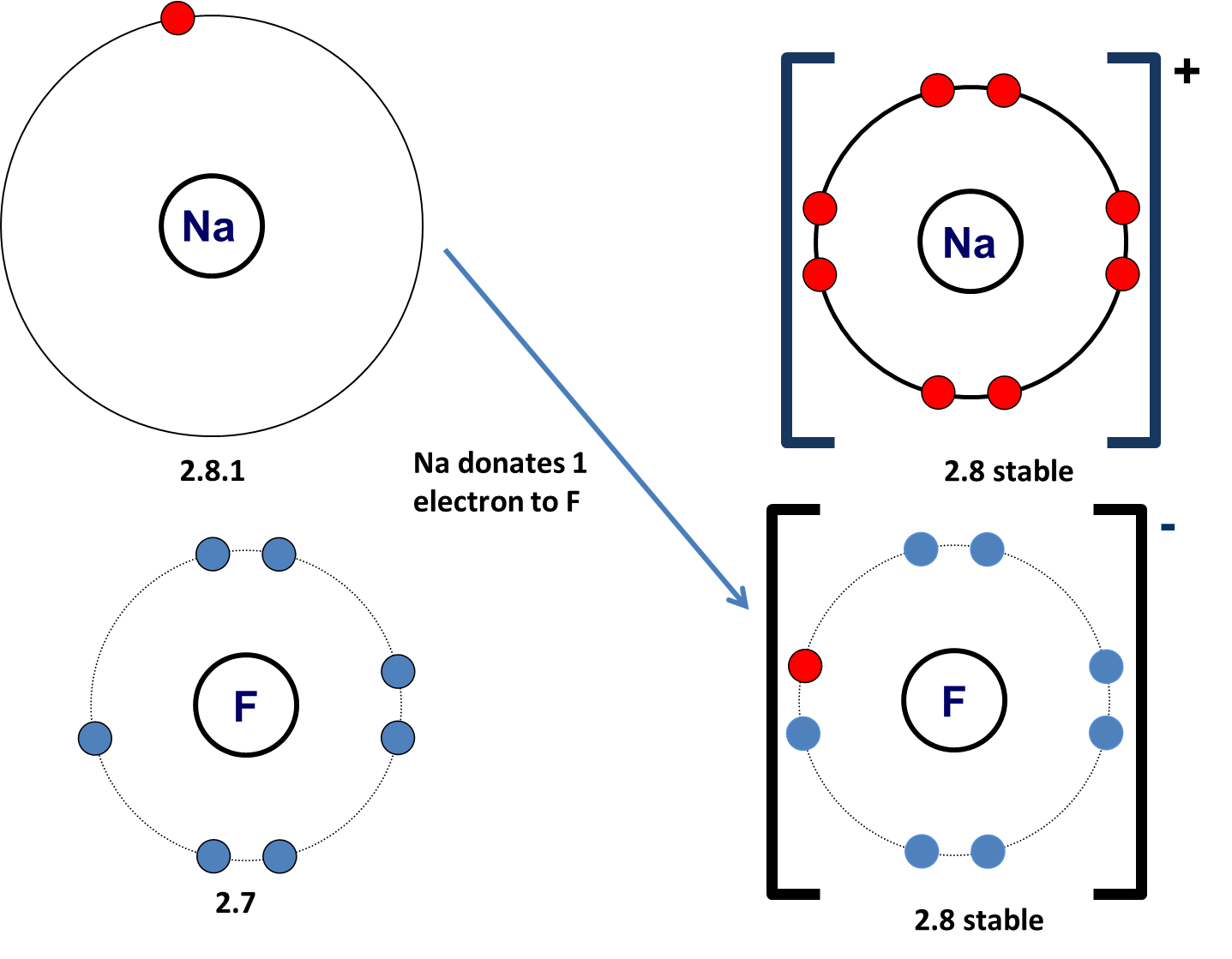
If they lose electrons they gain a positive charge as they have more protons than electrons. Sodium (2.8.1) loses one electron to become sodium ion (2.8) with a stable electronic configuration similar to Neon.
If they gain electrons they have more electrons than protons so, therefore, gain a negative charge. Chlorine (2.8.7) gains an electron to form chloride ion (2.8.8) with a stable electronic configuration similar to Argon.
Sodium fluoride is an ionic compound formed by the reaction between the metal sodium and the non-metal fluorine.
Sodium + fluorine ——> sodium fluoride
During chemical bonding metals and non-metals form ions and the charge (oxidation number) on an ion can be found using these steps:
- the number of charges on an ion formed by a metal is equal to the group number of the metal e.g. sodium is in group 1 and it's charge will be +1.
- the number of charges on an ion formed by a non-metal is equal to the group number minus eight e.g. oxygen is in group 6 and it's charge will be 6-8 = -2.
Ions are held together by very strong electrostatic forces between the oppositely charged ions, which is equal in all directions (omnidirectional) – allowing the ions to be held together very strongly.
They have very strong intermolecular bonds which gives them very high melting and boiling points.
The bonds between the ions form an arrangement called a giant ionic structure, which is very regular.
Covalent bonding
Bonding in which a pair of electrons, one from each atom, is shared between two non-metal atoms.
Covalent compounds are formed when NON-METAL atoms react together.
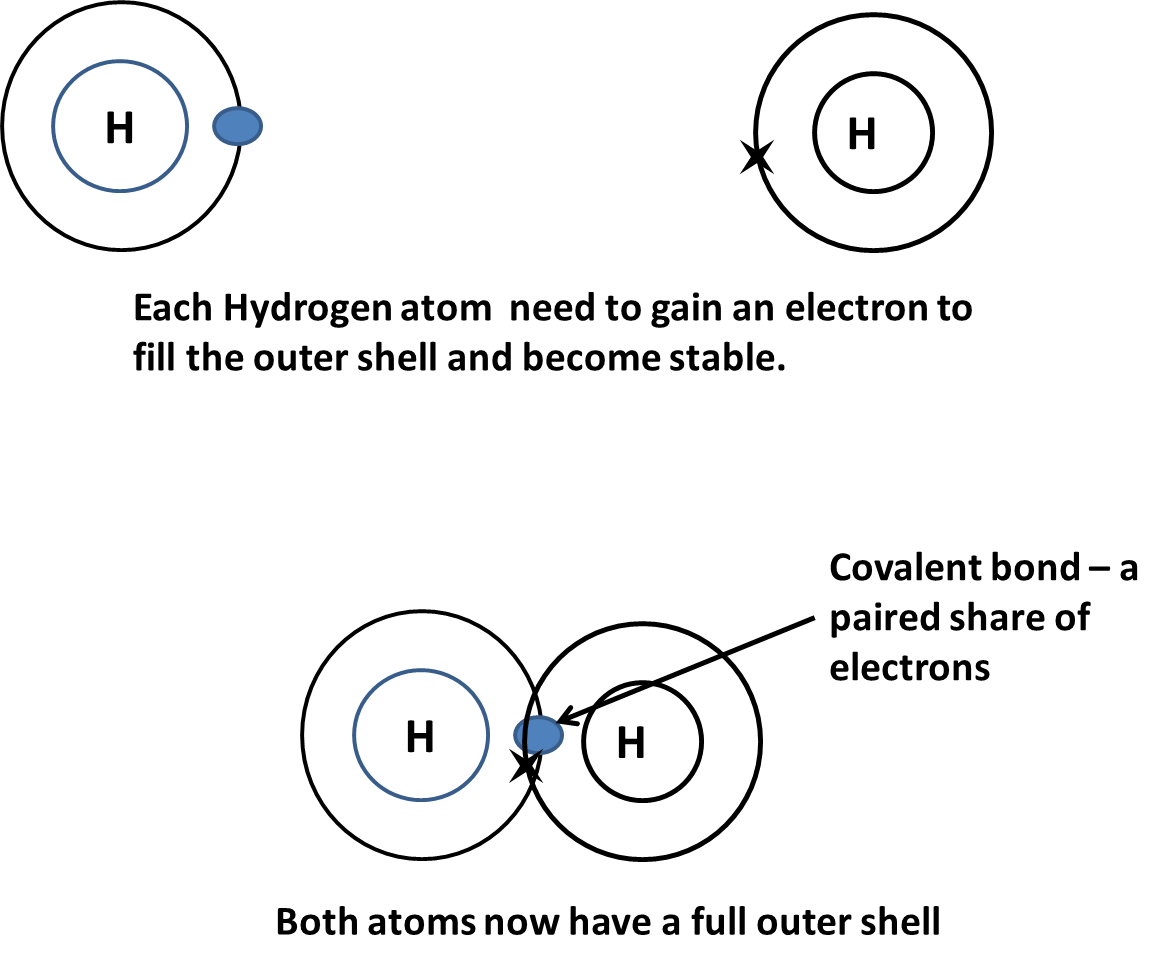
The strong electrostatic force of attraction between the bonding pair of electrons and the nucleus is called the covalent bond.
The inner shells do not always have to be included in diagrams as only the outer shell of electrons is involved in covalent bonding.
Many non-metal elements exist as molecules, which is two or more atoms joined by a covalent bond. Each atom has a full outer electron shell and is therefore stable.
Covalent structures
Covalent bonding can take place between atoms of different elements to create molecules of covalent compounds with different structures.
Covalent bonds in compounds can be single, double or triple.
Simple molecules
Oxygen, water and carbon dioxide are molecules as they only contain a few atoms which give them simple structures. Majority of molecular substances are gas or liquid at room temperature. However, a few are solid and these are called molecular solids.
The forces of attraction (Van der Waals forces) between molecules in molecular solids are weak and only require a small amount of energy to be broken. Therefore molecular solids have:
- have low melting and boiling points
- are usually soft and brittle
- are usually insoluble in water but soluble in other solvents such as hexane
- cannot conduct electricity as there are no free electrons to carry an electrical charge.
Giant covalent structures
In certain substances, thousands of atoms join together by covalent bonding producing giant covalent structures. All the bonds in giant covalent structures are covalent, which means gives them very high melting and boiling points. In addition, almost all giant covalent structures are hard but brittle.
Carbon allotropes
In carbon the atoms can bond in different ways to create different kinds of giant structures (diamond and graphite). These are called allotropes of carbon. In general, allotropes have the same chemical properties due to the same number of electrons. However, allotropes have different physical properties because the electrons are shared in different ways with other atoms.
Diamond
In diamond, all the electrons in the outer shell of the carbon atom (4) are used in covalent bonds.
Diamond is very hard
Diamond has a very high melting and boiling point therefore a lot of energy is needed to break the covalent bonds.
Diamond cannot conduct electricity as there are no free electrons or ions to carry an electrical charge.
Graphite
In graphite, only three of the four electrons in the outer shell of the carbon atom (4) are used in covalent bonds.
Graphite is soft and slippery because layers can easily slide over each other because the weak forces of attraction are easily broken.
Graphite can conduct electricity and is the only non-metal to do so. There is a free electron (delocalised) from each atom to carry a charge.
Metallic bond
Metal are found at the left and centre of the periodic table.
In metals, the outer electrons separate from the atoms to become delocalized and creating a ‘sea of electrons’. The atoms become positive ions and are attracted to these delocalised electrons and this attraction is called the metallic bond.
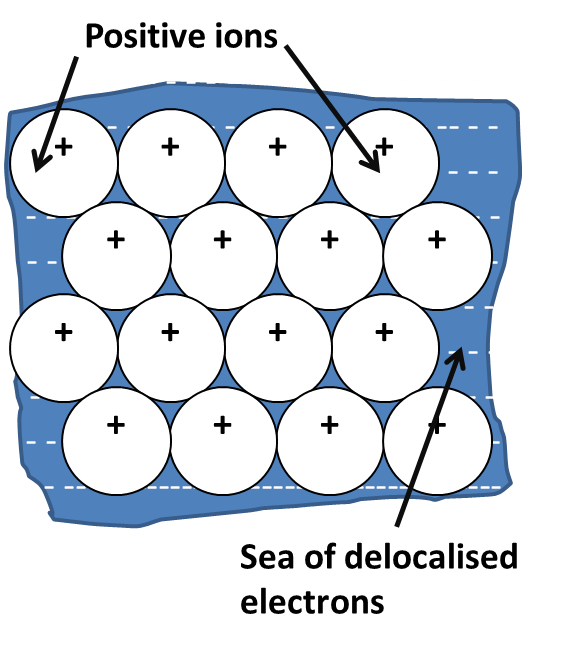
In metallic bonding, the atoms are tightly packed together in a giant lattice.
Properties of metallic bonds
Metal ions form a lattice which is more tightly packed and denser and form crystals called grains.
In general, metals have a very high melting and boiling point because metallic bonds are very strong and so a large amount of energy is needed to break them.
Metals are good conductors of heat as the free electrons can take in heat energy, which makes them move faster. The electrons can then transfer the energy throughout the lattice.
Metals are good conductors of electricity as the free delocalised electrons can carry an electrical charge.
Metals are malleable that is they can be bent and pressed into shape and ductile as they can be drawn out into wires. The layers of the lattice slide over each other when a force is applied and the bonds do not break because the electrons are free to move.
Metals can be made strengthened by adding another element to form an alloy. An alloy is a mixture of different elements. In alloys the atoms of the added element are larger which make it difficult for layers of metal atoms to slide. This makes the metal less malleable and less ductile
What you need to be able to do:
Be able to write a formula for an ionic compound given the charges and symbols
Be able to draw simple ‘dot & cross’ diagrams to represent covalent bonds
Be able to draw diagrams of metallic structures showing metal cations and delocalised electrons
Be able to relate the group number to the charge on an ion for example; aluminium is in group 3 so it has a 3+ charge on its ion
Ions are formed when atoms lose or gain electrons
Ions have the electronic structure of a noble gas
Know that compounds are substances in which atoms of two or more elements are chemically combined
Know that chemical bonding involves either the transfer of electrons (ionic) or the sharing of them (covalent)
Know that the electrons in a metallic structure are delocalised throughout the structure and are attracted to the metal cations by a strong electrostatic attraction
Be able to recognise simple molecules and giant structures from diagrams that show their bonding
Know that the ions are attracted to more than just one oppositely ion, the forces act in all directions